![]() TOC |
ID
|
Bioterrorism
| Anthrax
TOC |
ID
|
Bioterrorism
| Anthrax
![]()
Anthrax Detail see Anthrax | Medscape News on Anthrax | UpToDate Anthrax
REF:
CDC
Frequent Ask Questions about Anthrax |
Resouces on Anthrax & Bioterrorism
| Definition | Diagnosis | Reporting |
| Signs & Symptoms | Preventive Treatment | Response |
| Exposure | Treatment | Worker Safety |
| Testing | Vaccine | Source about Anthrax |
What is anthrax?
Bacillus anthracis, the etiologic agent of anthrax, is a large, gram-positive, non-motile, spore-forming bacterial rod. The three virulence factors of B. anthracis are edema toxin, lethal toxin and a capsular antigen. Human anthrax has three major clinical forms: cutaneous, inhalation, and gastrointestinal. If left untreated, anthrax in all forms can lead to septicemia and death.
What is the case definition for anthrax?
A confirmed case of anthrax is defined as
Epidemiology:
Symptoms of disease vary depending on how the disease was contracted, but symptoms usually occur within 7 days.
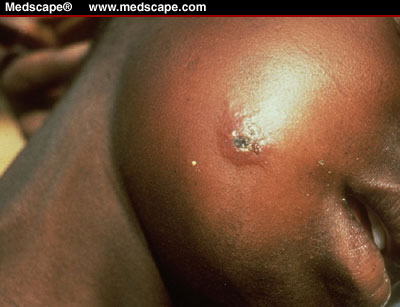
Cutaneous anthrax
is the most common naturally occurring type of infection (>95%) and usually
occurs after skin contact with contaminated meat, wool, hides, or leather
from infected animals. The incubation period ranges from 1-12 days. The skin
infection begins as a small papule, progresses to
a vesicle in 1-2 days followed by a necrotic ulcer. The lesion
is usually painless, but patients also may have
fever, malaise, headache, and regional lymphadenopathy. Most (about
95%) anthrax infections occur when the bacterium enters a cut of abrasion
on the skin. Skin infection begins as a raised bump that resembles a spider
bite, but (within 1-2 days) it develops into a vesicle and then a painless
ulcer, usually 1-3 cm in diameter, with a characteristic black necrotic (dying)
area in the center. Lymph glands in the adjacent area may swell. About 20%
of untreated cases of cutaneous anthrax will result in death. Deaths are
rare if patients are given appropriate antimicrobial therapy.
[[
Images
From the Dermatology Atlas: Cutaneous Anthrax
Would you recognize cutaneous anthrax? From Medscape
Dermatology.
 Explanation
]]
Explanation
]]
Inhalational anthrax
is the most lethal form of anthrax. Anthrax spores must be aerosolized in
order to cause inhalational anthrax. Studies show that 4,000 – 5,000
spores must be present to cause an infection. The incubation period of
inhalational anthrax among humans is unclear, but it is reported to range
from 1 to 7 days, possibly ranging up to 60 days. It resembles a viral
respiratory illness and initial symptoms include sore throat, mild fever,
muscle aches and malaise. These symptoms may progress to respiratory failure
and shock with meningitis frequently developing.
Gastrointestinal anthrax
usually follows the consumption of raw or undercooked contaminated meat and
has an incubation period of 1-7 days. It is associated with severe abdominal
distress followed by fever and signs of septicemia. The disease can take
an oropharyngeal or abdominal form.
Involvement of the pharynx is usually characterized by lesions at the base of the tongue, sore throat, dysphagia, fever, and regional lymphadenopathy. Oropharyngeal anthrax is associated with cervical edema and necrosis. A lesion, resembling a cutaneous anthrax lesion, may be seen in the oral cavity on the posterior wall, the hard palate or the tonsils. Patients typically complain of fever, dysphagia and lymphadenopathy.
Lower bowel inflammation usually causes nausea, loss of appetite, vomiting and fever, followed by abdominal pain, vomiting blood, and bloody diarrhea. The patient may present with the findings of an acute surgical abdomen.
Toxemia, shock and cyanosis characterize the terminal stages of both forms of the disease. The case fatality rate for gastrointestinal anthrax ranges from 25 to 60%.
Anthrax Meningitis:
Meningitis occurs in less than 5% of cases, and may be a complication of
any form of anthrax (inhalational, gastrointestinal or cutaneous). Rarely
does it occur without a primary focus. It is usually hemorrhagic.
Incubation period - concurrent with or one to several days after the onset of cutaneous, inhalation or gastrointestinal anthrax.
Symptoms - abrupt onset of meningeal symptoms including nausea, vomiting, myalgia, chills and dizziness. Laboratory findings are notable for a hemorrhagic meningitis.
Encephalomyelitis and cortical hemorrhages have been reported; death occurs in 1-6 days.
What specific symptoms should I watch for?
Persons should watch for the following symptoms:
Symptoms - Typically biphasic illness
Characteristic findings of acute phase Sx include:
x-ray findings:
Shock develops rapidly, sometimes accompanied by evidence of hemorrhagic meningitis, and patients usually die within 24 hours of onset of the acute phase. In prior outbreaks, mortality rates approached 90% despite appropriate antibiotic therapy.
The differential diagnosis of acute mediastinitis includes: esophageal perforation; trauma; contiguous spread from a head, neck or thoracic infection; and post-surgical infections after cardiothoracic procedures. Anthrax should be strongly considered in any previously healthy patient with acute mediastinitis.]]
Is anthrax contagious?
What are the case fatality rates for the various forms of anthrax?
Early treatment of cutaneous anthrax is usually curative, and early treatment of all forms is important for recovery. Patients with cutaneous anthrax have reported case fatality rates of 20% without antibiotic treatment and less than 1% with it. Although case-fatality estimates for inhalational anthrax are based on incomplete information, the rate is extremely high, approximately 75%, even with all possible supportive care including appropriate antibiotics. Estimates of the impact of the delay in post-exposure prophylaxis or treatment on survival are not known. For gastrointestinal anthrax, the case-fatality rate is estimated to be 25%-60% and the effect of early antibiotic treatment on that case-fatality rate is not defined.
What is the difference between exposure to anthrax and disease caused by anthrax?
A person can be said to be exposed to anthrax when that person comes in contact with the anthrax bacteria and a culture taken from that person is positive for anthrax. A person can be exposed without having disease. A person who might have come in contact with anthrax, but without a positive culture would be said to be potentially exposed. Disease caused by anthrax occurs when there is some sign of illness, such as the skin lesion that occurs with cutaneous anthrax.
A person who is exposed to anthrax but is given appropriate antibiotics can avoid developing disease.
Can I be exposed to anthrax via mail?
What kind of mail should be considered suspicious?
Some characteristics of suspicious packages and envelopes include the following:
Inappropriate or unusual labeling
Appearance
Other suspicious signs
If a package or envelope appears suspicious, DO NOT OPEN IT !!!
What should people do who get a letter of package with powder?
Handling of Suspicious Packages or Envelopes*
*These recommendations were published on October 26, 2001, in
“Update: Investigation of bioterrorism-related anthrax and interim
guidelines for exposure management and antimicrobial therapy.” MMWR
2001;50:909-919
Can anthrax spores be killed on letters in the mail by microwave, UV light, or ironing?
While some of these methods may kill some spores, it is not known what procedures to use (e.g., length of time, temperature, etc.). Furthermore, because of insufficient data on the efficacy of these methods in inactivating anthrax spores, CDC does not recommend these techniques for reliable decontamination.
There is no screening test for anthrax; there is no test that a doctor can do for you that says you’ve been exposed to or carry it. The only way exposure can be determined is through a public health investigation. The tests that you hear or read about, such as nasal swabs and environmental tests, are not tests to determine whether an individual should be treated. These kinds of tests are used only to determine the extent of exposure in a given building or workplace.
If the patient is suspected of being exposed to anthrax, should he/she be quarantined or should other family members be tested?
Does CDC collect samples to test the bacteria?
What's the turnaround time for an anthrax test in an environmental sample—for example, the time it takes to confirm that a substance in an envelope was indeed anthrax?
Does CDC recommend the use of home test kits for anthrax?
Hand-held assays (sometimes referred to as “Smart Tickets”) are sold commercially for the rapid detection of Bacillus anthracis. These assays are intended only for the screening of environmental samples. First responder and law enforcement communities are using these as instant screening devices and should forward any positive samples to authorities for more sensitive and specialized confirmatory testing. The results of these assays should not be used to make decisions about patient management or prophylaxis. The utility and validity of these assays are unknown. At this time, CDC does not have enough scientific data to recommend the use of these assays.
The analytical sensitivity of these assays is limited by the technology, and data provided by manufacturers indicate that a minimum of 10,000 spores is required to generate a positive signal. This number of spores would suggest a heavy contamination of the area (sample). Therefore a negative result does not rule out a lower level of contamination. Data collected from field use also indicate specificity problems with some of these assays. Some positive results have been obtained with spores of the non-anthrax Bacillus bacteria that may be found in the environment.
For these reasons, CDC has been asked to evaluate the sensitivity and specificity of the commercially available rapid, hand-held assays for B. anthracis. When this study is completed, results will be made available. Conclusions from this study are not expected in the near future.
How is anthrax diagnosed?
In patients with symptoms compatible with anthrax, providers should confirm the diagnosis by obtaining the appropriate laboratory specimens based on the clinical form of anthrax that is suspected (i.e., cutaneous, inhalational, or gastrointestinal).
What are the standard diagnostic tests used by the laboratories?
[[
When is a nasal swab indicated?
Nasal swabs and screening may assist in epidemiologic investigations, but should not be relied upon as a guide for prophylaxis or treatment. Epidemiologic investigation in response to threats of exposure to B. Anthracis may employ nasal swabs of potentially exposed persons as an adjunct to environmental sampling to determine the extent of exposure.
What is the therapy for preventing inhalational anthrax?
[[Prophylaxis:
Patient Isolation:
Universal precautions. Patients do not require isolation rooms.]]
What is cipro (ciprofloxacin)?
Does ciprofloxacin have an expiration date?
Yes. Antibiotics, just like all medicines, have expiration dates. If you received your ciprofloxacin through a pharmacist, the expiration date should be listed on the bottle. If you can’t find it or have questions about the expiration date, contact your pharmacist directly.
What are the side effects of Cipro?
What are the guidelines for changing from ciprofloxacin to another antibiotic?
Should people buy and store antibiotics?
[[Treatment:
The key to successful treatment is prompt administration of an antimicrobial at the first suspicion of anthrax.
Treatment for Non-Pregnant Adults:
Rx for Inhalation anthrax (this regimen also recommended for gastrointestinal and meningeal anthrax)
For penicillin resistant anthrax:
For penicillin susceptible anthrax:
Supportive therapy is often required (e.g., volume expanders, vasopressor agents and oxygen). A tracheotomy may be needed if cervical edema compromises the airways.]]
What is the treatment for patients with inhalational and cutaneous anthrax?
What if I develop side effects from the antibiotic?
If you develop side effects from the antibiotic, call your health care provider immediately. Depending on the type of side effects, you may be able to continue taking the medicine, or may be switched to an alternative antibiotic. If necessary, your physician may contact your State Department of Health for consultation on possible alternate antibiotics.
Has CDC tested the anthrax isolates for sensitivity to different antibiotics?
What are the risks of using tetracyclines and fluoroquinolones in children; are alternatives available?
Risks of using tetracyclines and fluroquinolones in children must be weighed carefully against the risk for developing a life-threatening disease due to B. anthracis. Both agents can have adverse health reactions in children. If adverse reactions are suspected, therapy may be changed to amoxicillin or penicillin.
Is the anthrax vaccine available to the public?
Who should be vaccinated against anthrax?
The Advisory Committee on Immunization Practices (ACIP) has recommend anthrax vaccination for the following groups:
What is the protocol for anthrax vaccination?
Are there adverse reactions to the anthrax vaccine?
Mild local reactions occur in 30% of recipients and consist of slight tenderness and redness at the injection site. Severe local reactions are infrequent and consist of extensive swelling of the forearm in addition to the local reaction. Systemic reactions occur in fewer than 0.2% of recipients.
Physicians should report any suspected cases of B. anthracis to their local or state public health officials IMMEDIATELY. Subsequent notification procedures for these officials may be found on this Web Site at: http://www.bt.cdc.gov/EmContact/Protocols.asp
How is CDC responding to the anthrax reports?
What is CDC’s role on “rapid response teams”?
What is the approach to cleanup of buildings?
Does CDC cooperate with international health organizations like the World Health Organization (WHO) to help in other countries with anthrax cases?
What are CDC’s recommendations for protecting mail handlers?
What are CDC’s recommendations for protecting mail handlers?
CDC and the US Postal Service are collaborating to ensure that all mail handlers and postal workers are protected against exposure to anthrax. Detailed guidelines may be found on this Web Site at: http://www.bt.cdc.gov/DocumentsApp/Anthrax/10312001/han51.asp
How long do anthrax spores live?
What is the importance of knowing the genetic information about anthrax?
Genetic information about B. anthracis, particularly to determine genetic similarity among strains, is an important part of a disease investigation, but it is not immediately required for taking action to prevent or treat anthrax in those who may have been exposed to or infected by B. anthracis. Genetic information is often used to determine the similarity of strains if a common source is suspected.
Does the similarity in strains from Florida, New York, and Washington, D.C. mean that they came from the same source or are these just the most common strains?
In general all information presented in these pages and all items available for download are for public use. However, you may encounter some pages that require a login password and id. If this is the case you may assume that information presented and items available for download therein are for your authorized access only and not for redistribution by you unless you are otherwise informed.
Content related questions should be directed to: Content Administrator
Site related questions should be directed to: Webmaster
This page last reviewed: Nov. 3, 2001
Morbidity and Mortality Weekly Report October 2001;50:909-919
Update: Investigation of Bioterrorism-Related Anthrax and Interim Guidelines for Exposure Management and Antimicrobial Therapy
CDC Editorial Note:
Bioterrorism attacks using B. anthracis spores sent through the mail have
resulted in 15 anthrax cases and three deaths. The initial anthrax cases
occurred among persons with known or suspected contact with opened letters
contaminated with B. anthracis spores.
The possibility of further exposure to B. anthracis and subsequent clinical
illness exists. Clinicians and laboratorians should be vigilant for symptoms
or laboratory findings that indicate B. anthracis infection, particularly
among mail handlers. Information to guide health-care providers and laboratorians
is available at
http://www.bt.cdc.gov.
Managing Threats
Letters containing B. anthracis spores have been sent to persons in NYC and DC. Prompt identification of a threat and institution of appropriate measures may prevent inhalational anthrax. To prevent exposure to B. anthracis and subsequent infection, suspicious letters or packages should be recognized and appropriate protective steps taken.
Characteristics of suspicious packages and letters include inappropriate or unusual labeling, strange return address or no return address, postmarks from a city or state different from the return address, excessive packaging material, and others. If a package appears suspicious, it should not be opened. The package should be handled as little as possible. The room should be vacated and secured promptly and appropriate security or law enforcement agencies promptly notified (see sidebar).
Managing Exposures
Identification of a patient with anthrax or a confirmed exposure to B. anthracis should prompt an epidemiologic investigation. The highest priority is to identify at-risk persons and initiate appropriate interventions to protect them. The exposure circumstances are the most important factors that direct decisions about prophylaxis. Persons with an exposure or contact with an item or environment known, or suspected to be contaminated with B. anthracisregardless of laboratory tests resultsshould be offered antimicrobial prophylaxis. Exposure or contact, not laboratory test results, is the basis for initiating such treatment. Culture of nasal swabs is used to detect anthrax spores. Nasal swabs can occasionally document exposure, but cannot rule out exposure to B. anthracis. As an adjunct to epidemiologic evaluations, nasal swabs may provide clues to help assess the exposure circumstances. In addition, rapid evaluation of contaminated powder, including particle size and characteristics, may prove useful in assessing the risk for inhalational anthrax.
CDC is working with U.S. Postal Service employees and managers on several strategies to address the risk for anthrax among workers involved in mail handling. These strategies include personal protective equipment for workers handling mail and engineering controls in mail facilities. Clinicians and laboratorians should be vigilant for symptoms or laboratory findings that indicate possible anthrax infection, particularly among workers involved in mail sorting and distribution. Information to guide health-care providers and laboratories is available at http://www.bt.cdc.gov.1
Antimicrobial Treatment
A high index of clinical suspicion and rapid administration of effective antimicrobial therapy is essential for prompt diagnosis and effective treatment of anthrax. Limited clinical experience is available and no controlled trials in humans have been performed to validate current treatment recommendations for inhalational anthrax. Based on studies in nonhuman primates and other animal and in vitro data, ciprofloxacin or doxycycline should be used for initial intravenous therapy until antimicrobial susceptibility results are known (Table 1).
Because of the mortality associated with inhalational anthrax, two or more antimicrobial agents predicted to be effective are recommended; however, controlled studies to support a multiple drug approach are not available. Other agents with in vitro activity suggested for use in conjunction with ciprofloxacin or doxycycline include rifampin, vancomycin, imipenem, chloramphenicol, penicillin and ampicillin, clindamycin, and clarithromycin; but other than for penicillin, limited or no data exist regarding the use of these agents in the treatment of inhalational B. anthracis infection. Cephalosorins and trimethoprim-sulfamethoxazole should not be used for therapy. Regimens being used to treat patients described in this report include ciprofloxacin, rifampin, and vancomycin; and ciprofloxacin, rifampin, and clindamycin.
Penicillin is labelled for use to treat inhalational anthrax. However, preliminary data indicate the presence of constitutive and inducible beta-lactamases in the B. anthracis isolates from Florida, NYC, and DC. Thus, treatment of systemic B. anthracis infection using a penicillin alone (i.e., penicillin G and ampicillin) is not recommended. The B. anthracis genome sequence shows that this organism encodes two beta-lactamases: a penicillinase and a cephalosporinase. Data in the literature also show that some beta-lactamase negative B. anthracis strains for which the penicillin MICs are 0.06 µg/mL increase to 64 µg/mL and become beta-lactamase positive when exposed to semisynthetic penicillins.4 The frequency of this induction event is unknown. Although amoxicillin/clavulanic acid is more active than amoxicillin alone against beta-lactamase, producing strains in vitro, the combination may not be clinically effective for inhalational anthrax where large numbers of organisms are likely to be present.
Toxin-mediated morbidity is a major complication of systemic anthrax. Corticosteroids have been suggested as adjunct therapy for inhalational anthrax associated with extensive edema, respiratory compromise, and meningitis.5
For cutaneous anthrax, ciprofloxacin and doxycycline also are first-line therapy (Table 2). As for inhalational disease, intravenous therapy with a multidrug regimen is recommended for cutaneous anthrax with signs of systemic involvement, for extensive edema, or for lesions on the head and neck. In cutaneous anthrax, antimicrobial treatment may render lesions culture negative in 24 hours, although progression to eschar formation still occurs.5 Some experts recommend that corticosteroids be considered for extensive edema or swelling of the head and neck region associated with cutaneous anthrax. Cutaneous anthrax is typically treated for 7-10 days; however, in this bioterrorism attack, the risk for simultaneous aerosol exposure appears to be high. Although infection may produce an effective immune response, a potential for reactivation of latent infection may exist. Therefore, persons with cutaneous anthrax associated with this attack should be treated for 60 days.
Prophylaxis for inhalational anthrax exposure has been addressed in a previous report1 and indicates the use of either ciprofloxacin or doxycycline as first line agents. High-dose penicillin (e.g., amoxicillin or penicillin VK) may be an option for antimicrobial prophylaxis when ciprofloxacin or doxycycline are contraindicated. The likelihood of beta-lactamase induction events that would increase the penicillin MIC is lower when only small numbers of vegetative cells are present, such as during antimicrobial prophylaxis.
All medications may have undesirable side effects and allergic reactions may result from any medication. Clinicians prescribing these medications should be aware of their side effects and consult an infectious disease specialist as needed. Patients should be urged to inform their health-care provider of any adverse event.
This is the first bioterrorism-related anthrax attack in the United States, and the public health ramifications of this attack continue to evolve. Additional updates and recommendations will be published in MMWR.
Handling of Suspicious Packages or Envelopes
Resources on Bioterrorism & Anthrax
*** Website Resources for Bio-Terrorism
AMA Message
to Physicians on Anthrax
CDC - FAQs
about Anthrax
CDC
- How to handle Anthrax Threat 10-12-2001
CDC
- One Page Fact about Anthrax 10-14-2001
Anthrax
JAMA May 12, 1999
Anthrax - NY City
Dept. of Health Bio-Threat Website
Anthrax - Background (
CAnthrax BAckground
Center for Nonproliferation Studies )
Anthrax Review - Cleveland Clinic December 1999
NEJM
Review on Anthrax September 9, 1999
NEJM
Book Review on Anthrax May 4, 2000
11152001
|
Therapy |
Prophylaxis* |
|
Adult Doses |
Adult Doses |
Susceptibility Results Unknown or Penicillin- Resistant** |
Ciprofloxacin 400mg IV q 8- 12h |
Ciprofloxacin 500mg po bid |
Penicillin Susceptible |
Penicillin G 80,000 units per kg in 1st hour followed by 320,000 units/kg/day.
(Average adult dose is 4 million units q 4hr or 2 million units q
2h) |
Penicillin VK 30mg/kg/d in 4 divided doses |
|
Pediatric Doses |
Pediatric Doses |
Susceptibility results unknown or penicillin- resistant |
Ciprofloxacin 20-30mg/kg/day IV in 2 divided doses (maximum daily dose
not to exceed 1 gram/d) |
Ciprofloxacin 20-30mg/kg per day po divided in 2 doses |
Penicillin-susceptibility |
Penicillin G 250,000 units/kg per day IV administered every 4 hours |
Penicillin VK 30 mg/kg per day po administered in 4 divided doses |
Antibiotic prophylaxis should be continued for 60 days if anthrax vaccine is not available (or if vaccine is available, antibiotics should be continued until 3rd dose of vaccine has been administered).
In pregnant women, penicillin-resistant anthrax should be treated with ciprofloxacin. If doxycycline is used, liver function tests should be monitored closely.